 Hotline
Hotline
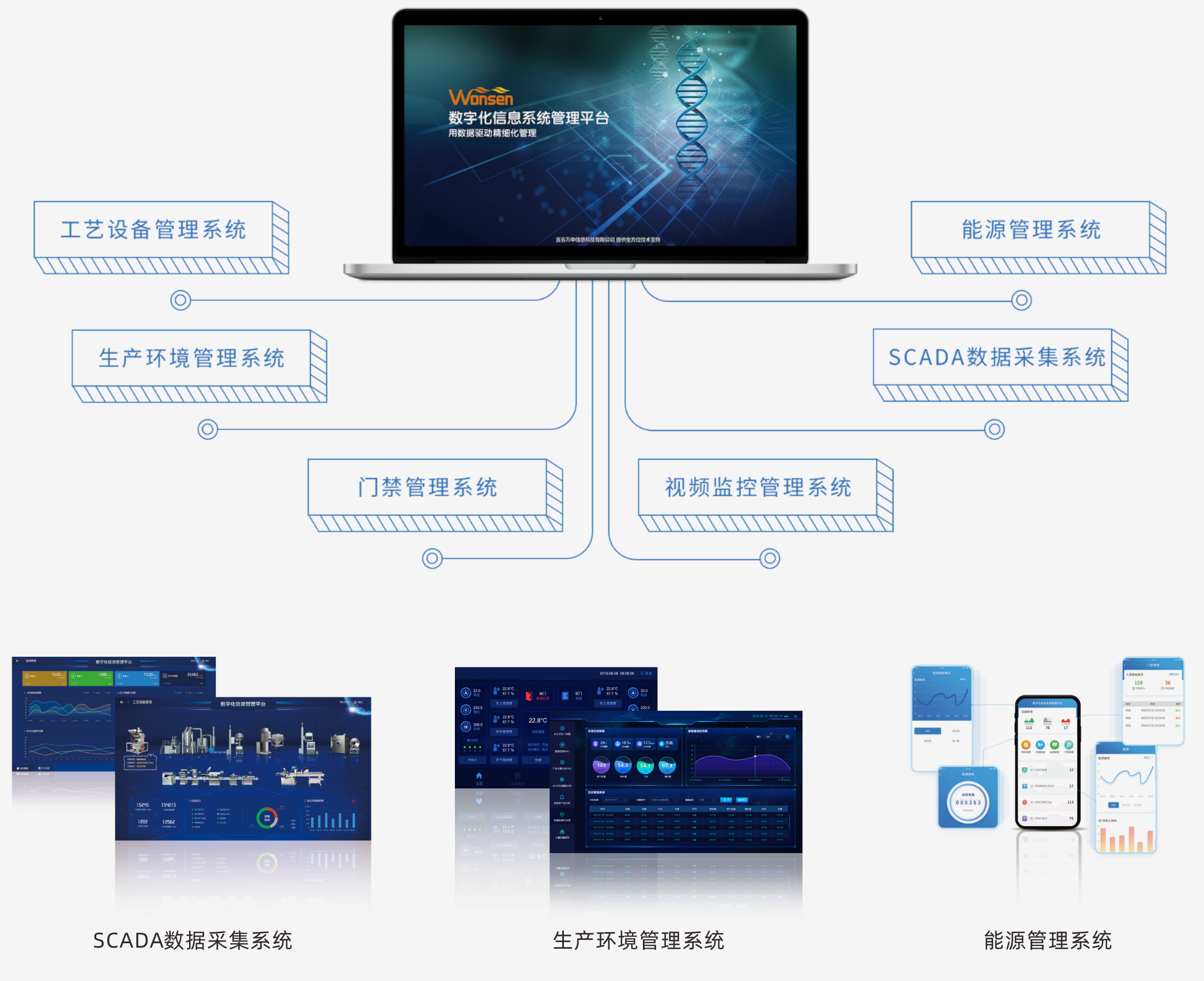
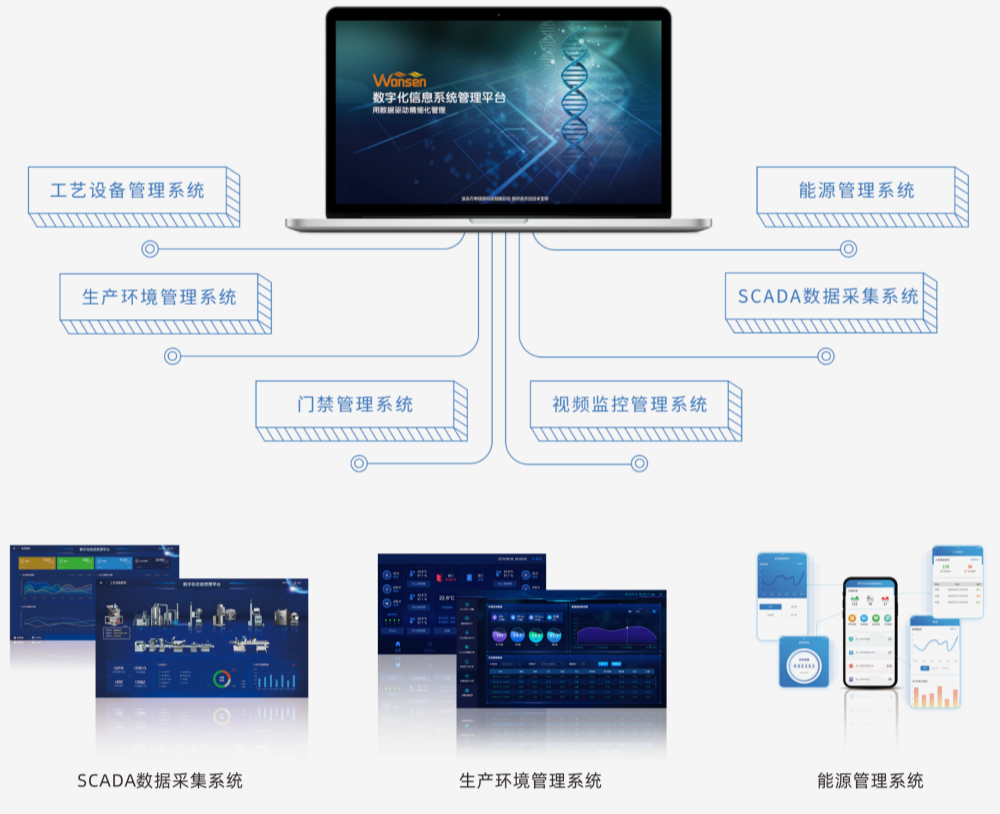
Digital information management system
Our system integrates process equipment and utility systems, consolidating operational, production, energy, and environmental data into a real-time database. This allows for data analysis, optimization, and visualization of the production process, creating centralized electronic batch records and enabling intelligent enterprise management.
Data Collection
Real-time data gathering and consolidation through gateways and industrial IoT platforms, supporting production monitoring and execution.
Customizable Reporting
Tailored reports to enhance enterprise management efficiency.
Real-time Production Monitoring
Instant insights into processing operations for swift decision-making.
Integration with Existing Systems
Seamless connection with ERP, MES, PDM, LIMS, and other systems to prevent data isolation.
Electronic Records Integrity
Ensures the authenticity, accuracy, and traceability of records.
Multi-platform Visualization
Supports PC, App, and large screen displays, catering to diverse managerial needs.
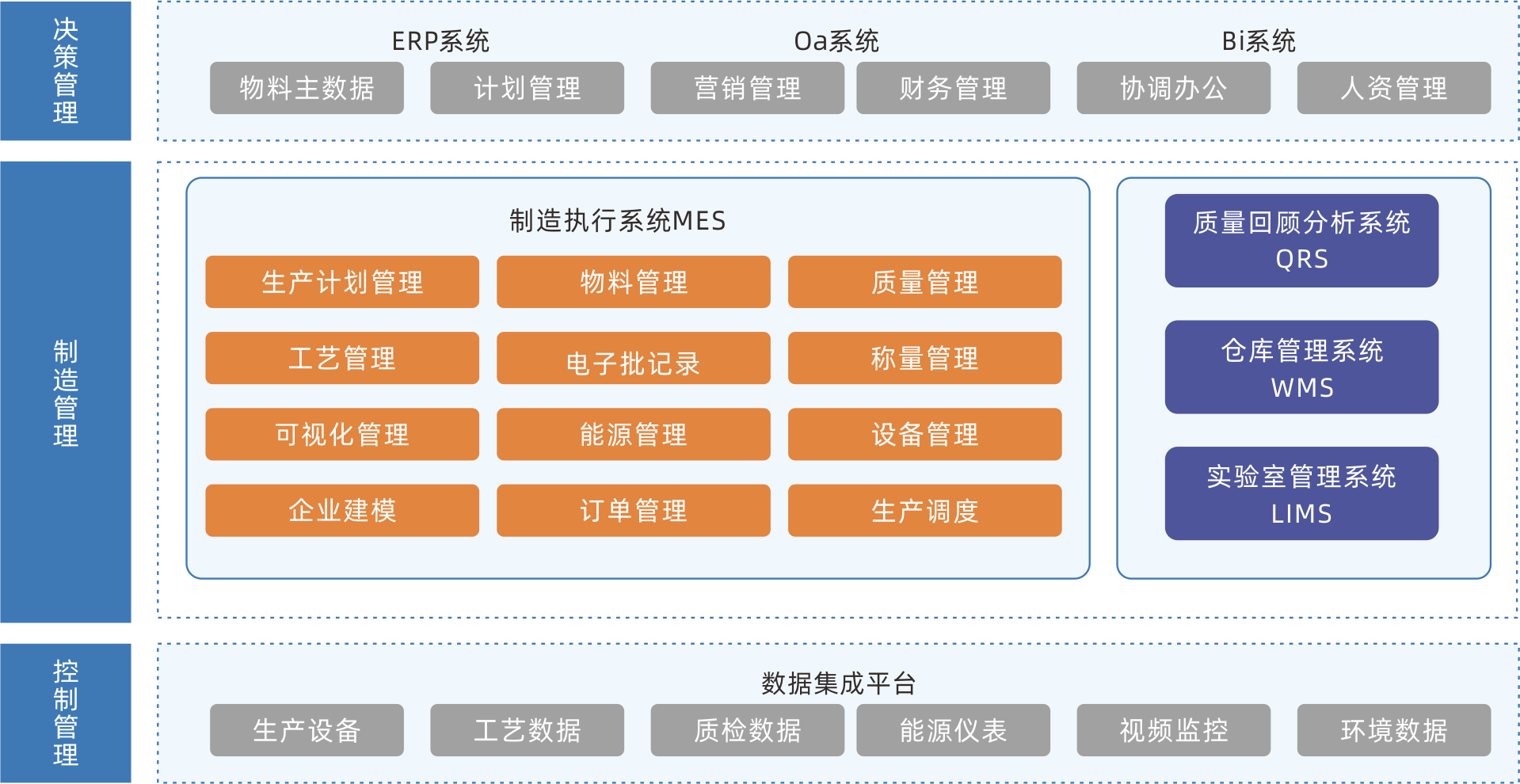
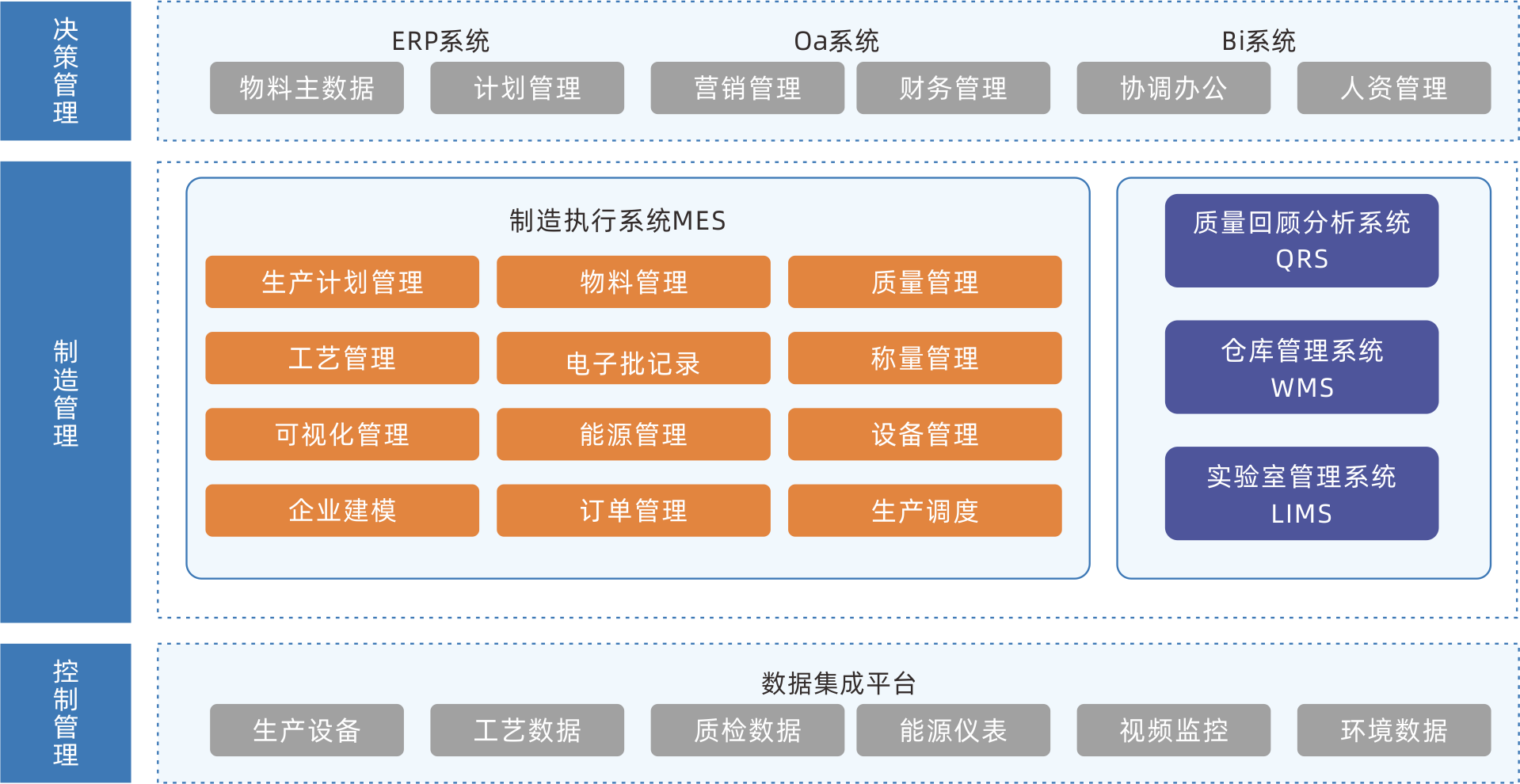
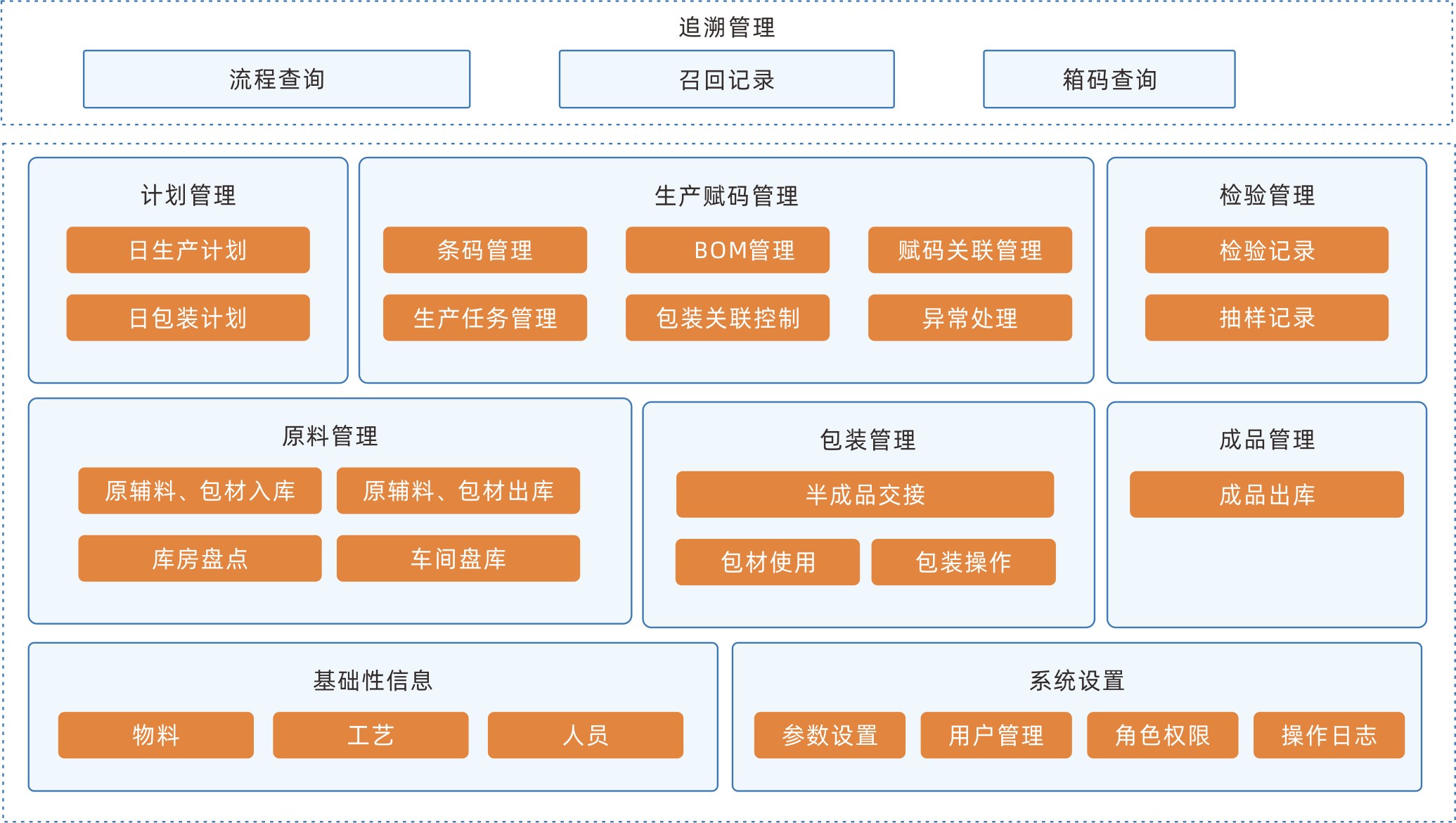
MES production execution management system
MES is a production information management system aimed at the execution layer of the entire manufacturing process. It connects enterprise resource management with workshop operation site control, and will truly become the production command center and production collaboration platform of the enterprise. The system covers management modules such as material management, formula management, planning and scheduling management, production scheduling management, inventory management, quality management, cost management, data analysis and decomposition, etc., creating a solid, reliable, and feasible manufacturing collaborative management platform for enterprises.
Continuously optimizing the production process, reducing production costs, shortening delivery cycles, and increasing production capacity
Information management of production lines, processes, production materials, production capacity, and other information helps enterprises achieve the goal of lean production and manufacturing.
Effectively preventing information distortion
By adopting transparent production monitoring technology, the entire production process is controllable, promoting the adjustment of production plans, preventing information distortion, and ensuring the real-time and accuracy of production information.
Provide basic data support for intelligent production scheduling
Being able to grasp all information during the production process in real-time, preparing for the subsequent upgrade of intelligent production scheduling.
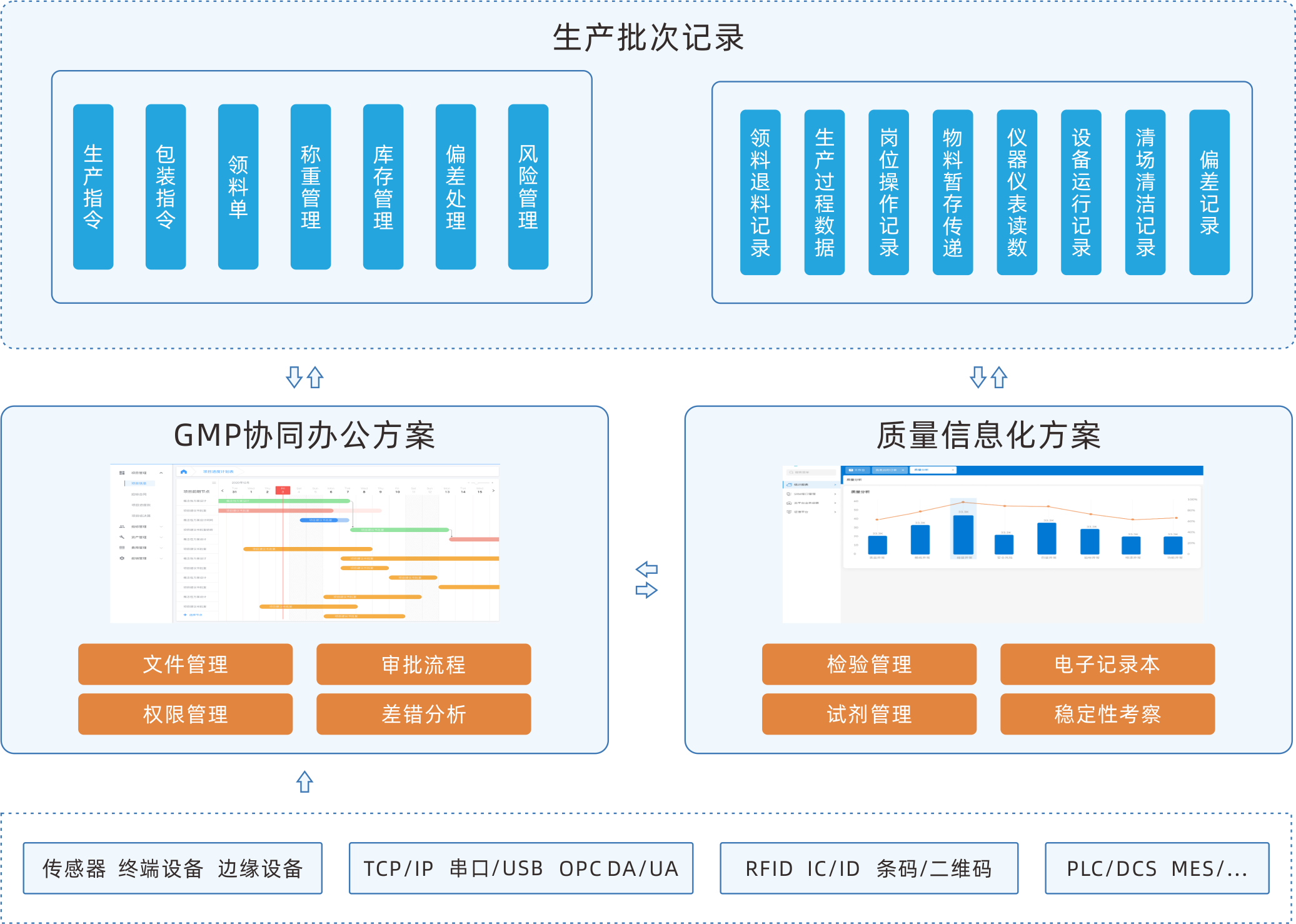
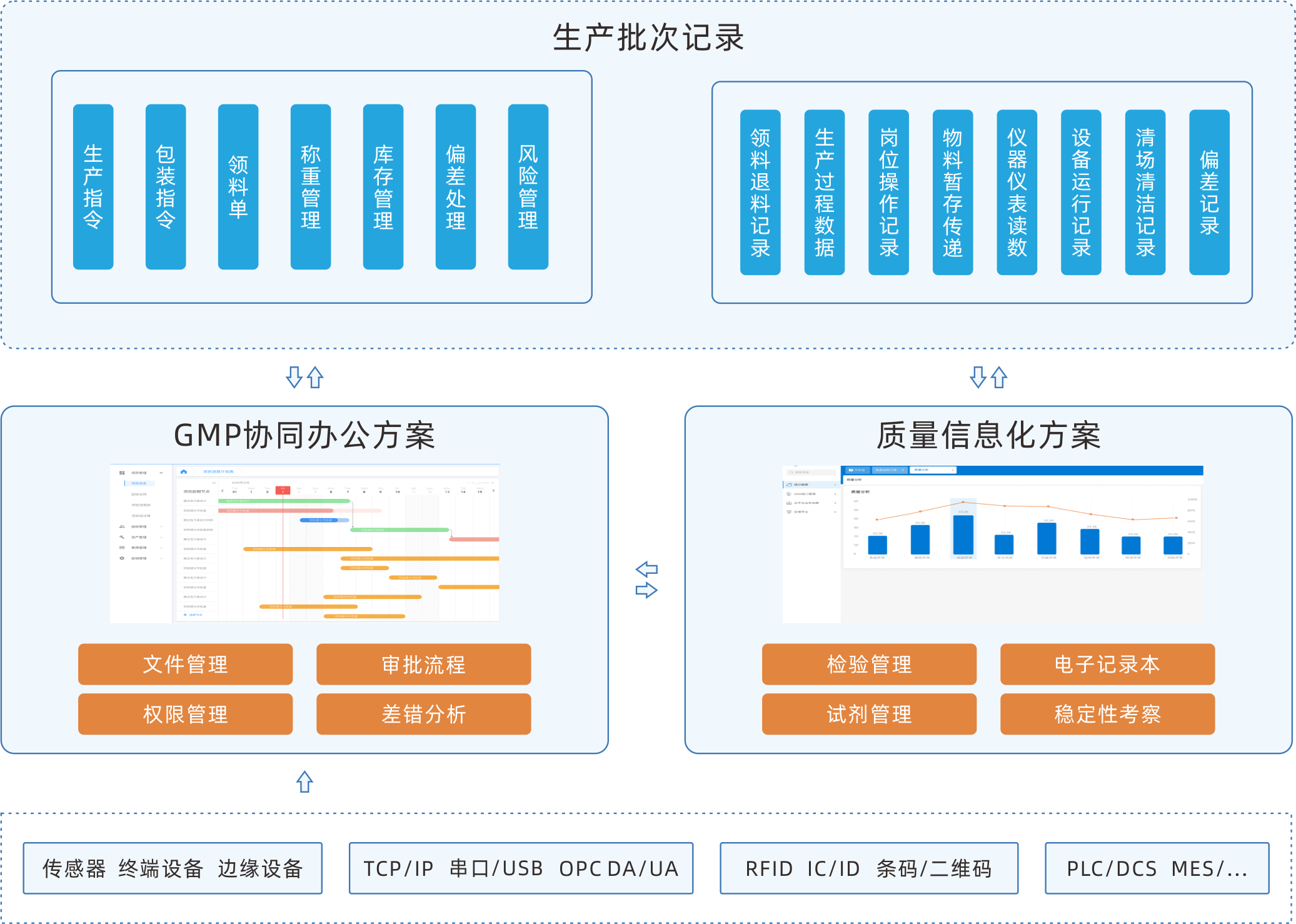
EBR electronic batch recording system
EBR software can execute electronic programs according to the standard operating procedures of production, adding real-time data of equipment, environment, etc. as a more comprehensive supplement to batch records. It collects, summarizes, classifies, and organizes.The sofeware integrates batch production records, batch packaging records, and batch inspection records into authentic, complete, accurate, safe, and efficient records, achieving paperless production in the production workshop.
Collaborative integration system
In addition to being deployed separately, it can serve as a separate module for a complete MES, interact data with other modules and third-party systems.
Efficient operational processes
Automatically display approved versions of all files and drawings required for each device and the operating SOP for each process. Automatically record users who have completed operations, and provide electronic signatures to accelerate the signing process.
Equipment data collection
Automatically collect PLC/SCADA system data and input them into batch records and save valuable time. Ensure 100% accuracy.
Automatic workflow
The preset workflow ensures the orderly progress of electronic batch record release work, with message reminders and automatic approval push.
Real time tracking and abnormal alarm
Real time monitoring of the collection and filling of electronic batch record data. Do real time verification by setting standard process parameters and collecting or filling in parameters When data is abnormal, abnormal information can be automatically generated according to the set rules, and production and quality personnel can conduct real-time deviation analysis and evaluation of the abnormality.
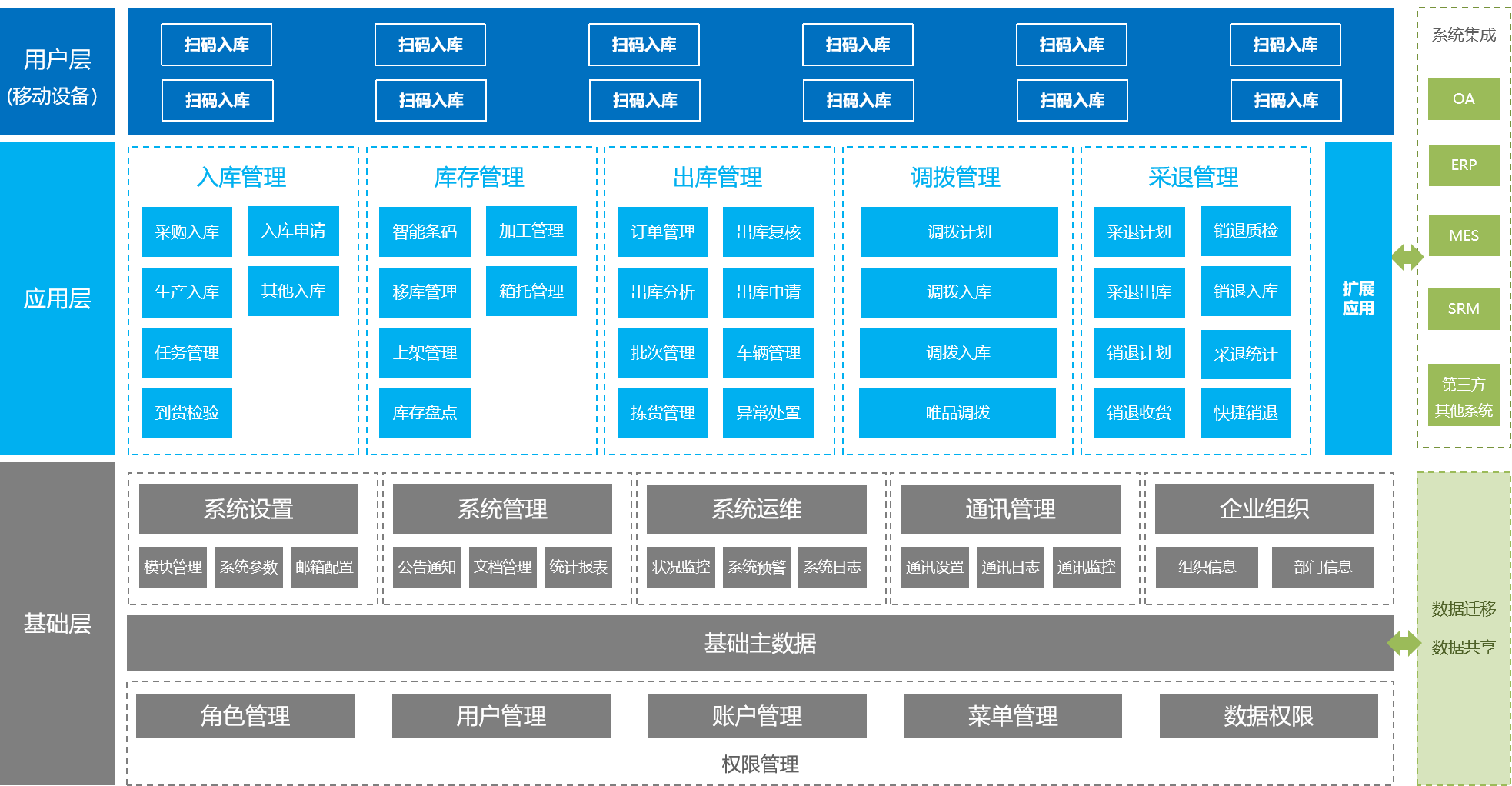
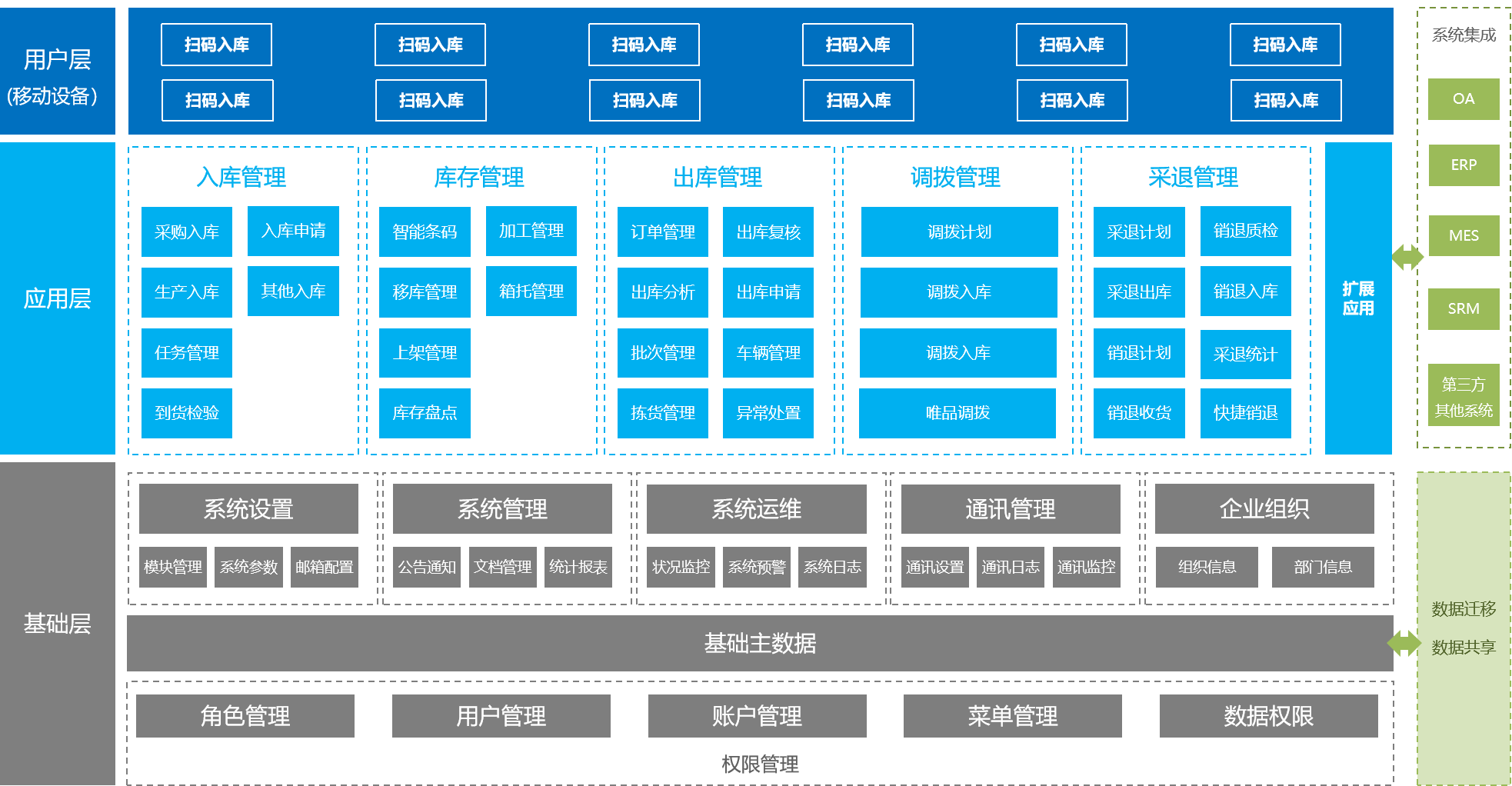
WMS warehouse management system
The WMS warehouse management system is a more refined management system for warehouses, effectively controlling and tracking the entire process of logistics and cost management in warehouse operations, and achieving comprehensive enterprise warehouse information management.
Improve inventory space utilization
Through real-time data collection and analysis at various stages, help managers make scientific decisions, improve warehouse management level, improve inventory accuracy, and reduce inventory costs.
Effectively preventing information distortion
Optimize the homework path, guide homework methods, and reasonably reduce logistics operation costs.
Provide basic data support for intelligent production scheduling
By utilizing the convenient processing and intelligent information technology of mobile devices, the demand for experience and skills of warehouse employees is reduced, effectively hiding labor costs.
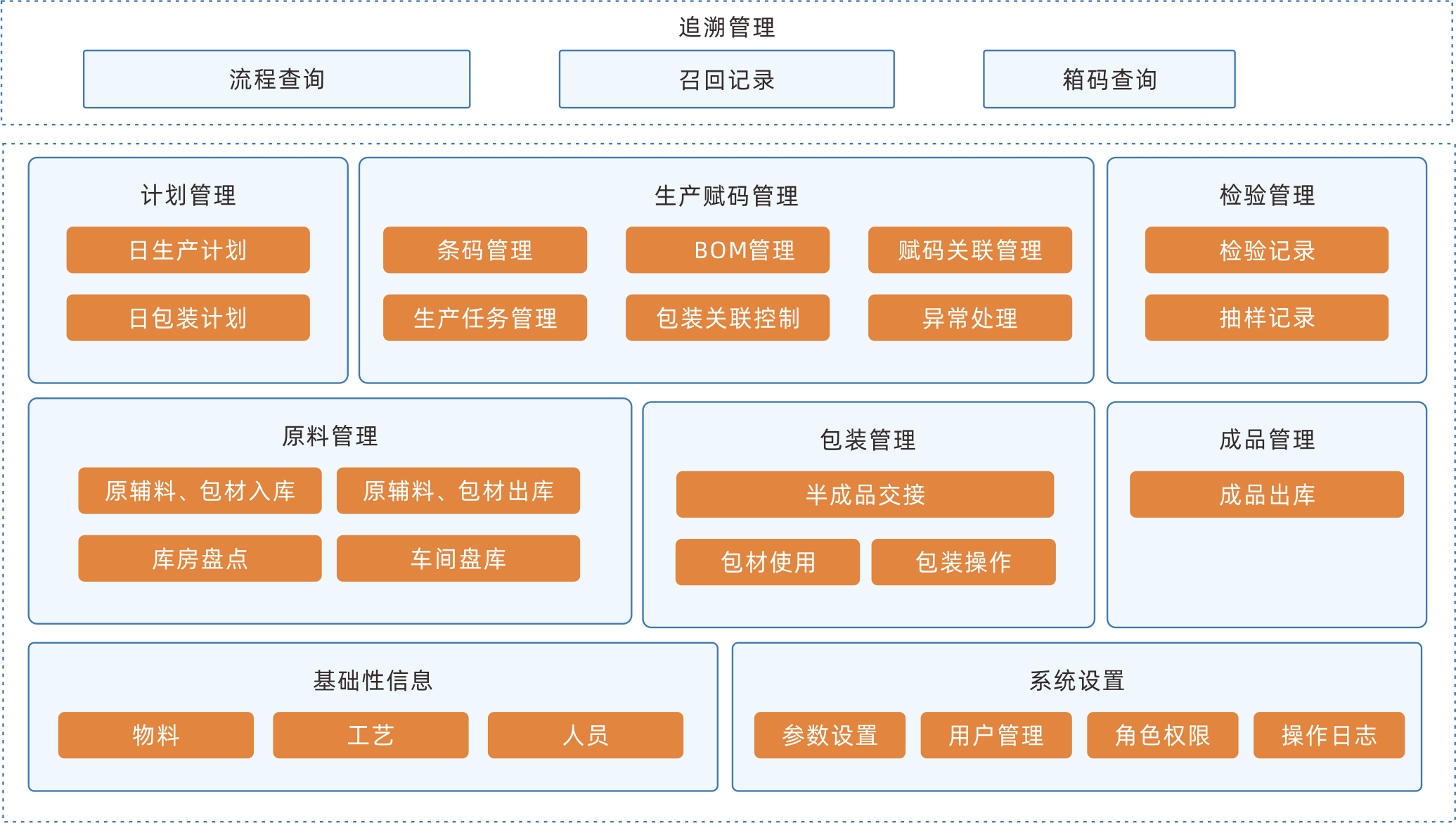
Information traceability system
The traceability system scans and marks all links of the product, including raw materials, production process, and circulation, establishing a traceability system to achieve product traceability management and control risks in each link.
Tracing one-stop services
Realize the full process visual management of raw material entry, product production, warehousing and logistics, terminal sales, and market consumption, and assist enterprises in quickly establishing a traceability system that meets national and market supervision.
Full process control of the supply chain
Improve the planning, coordination, operation, control, and optimization activities and processes of various links in the enterprise supply chain, and obtain real-time observation of the entire supply chain network.
One stop collaboration of data
Eliminating information silos, enterprise data can be interconnected, shared in real-time, and efficiently coordinated with upstream and downstream data throughout the entire industry chain.
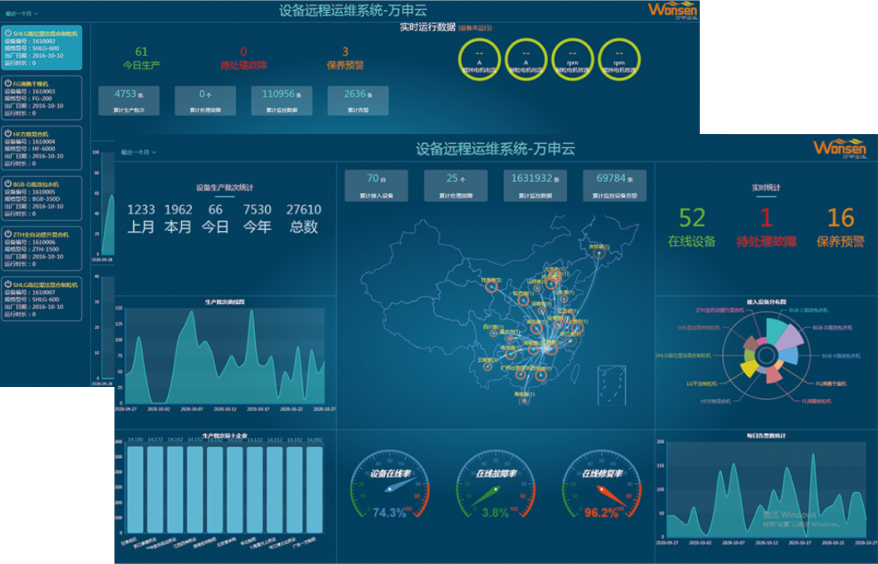
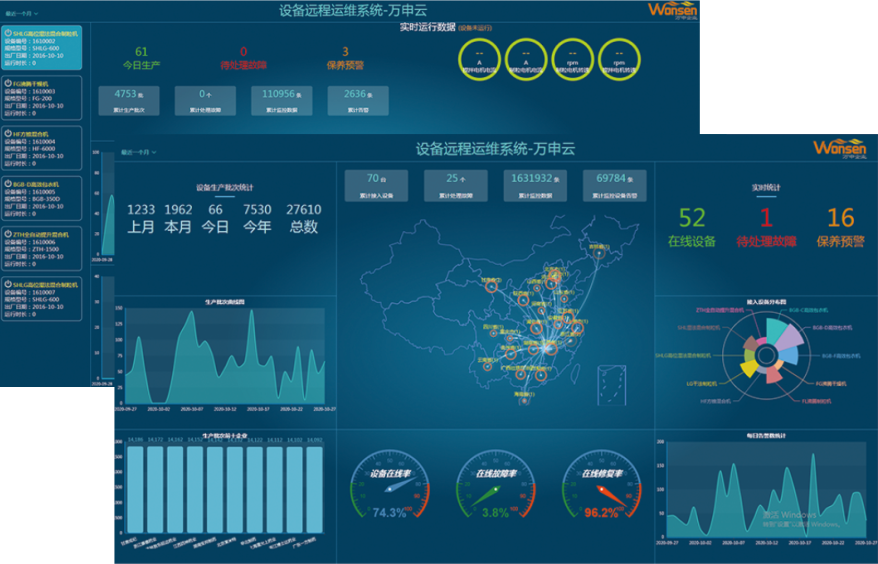
Remote operation and maintenance system
The remote maintenance system for equipment is a remote comprehensive management system based on data application cloud and intelligent gateway. The intelligent gateway is connected to the device end to automatically collect operational status and related data (geographic location, temperature and humidity, pressure, speed, flow rate, etc.), and send the information to the data application cloud platform. Assist enterprises in equipment management, monitoring, early warning, diagnosis, and maintenance work order management, provide customers with full lifecycle services for equipment, and achieve comprehensive improvement of enterprise asset efficiency.
Equipment maintenance
Plan development cycle task generation, completion rate statistics, and full process intelligence.
Equipment repair report
You can view the device's alarms and health status through the platform, and it also has the function of automatic alarm reminders.
Spare parts
Inventory inquiry, inventory warning, consumption statistics, and usage analysis.
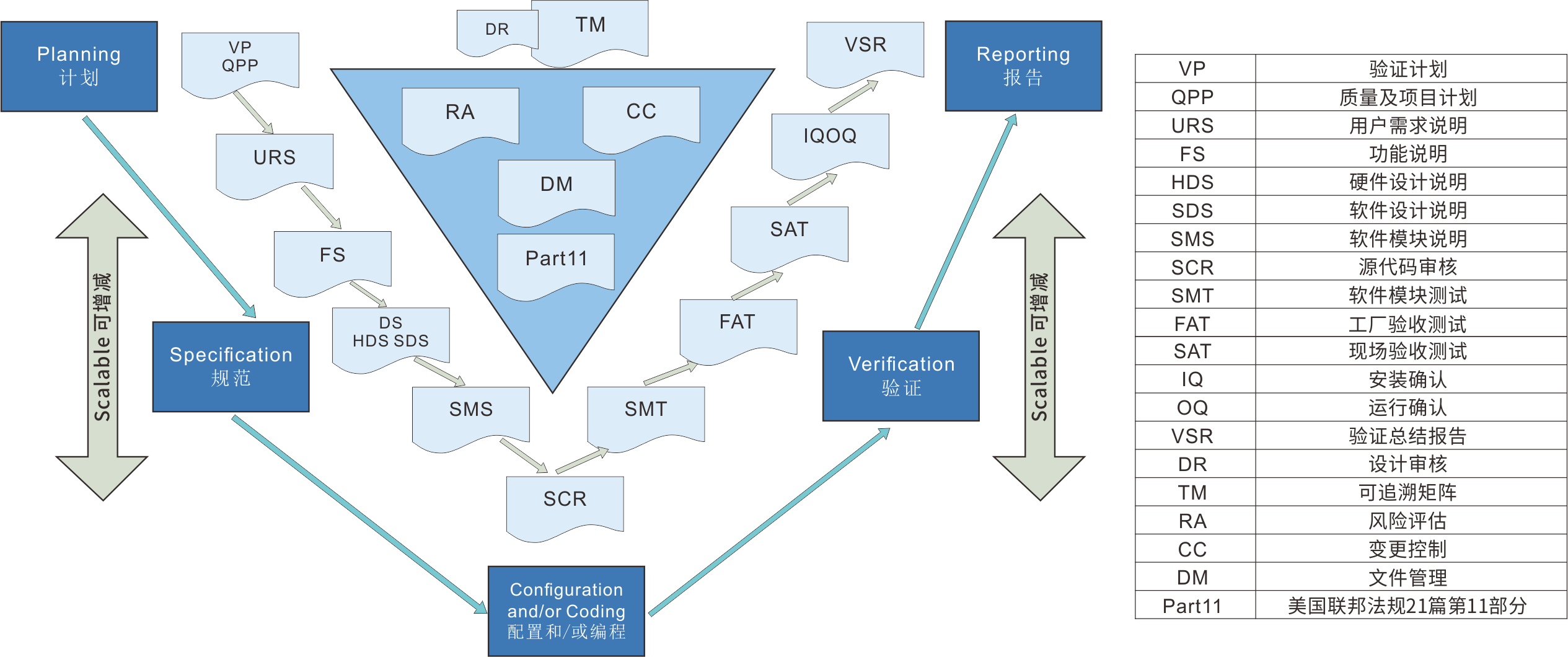
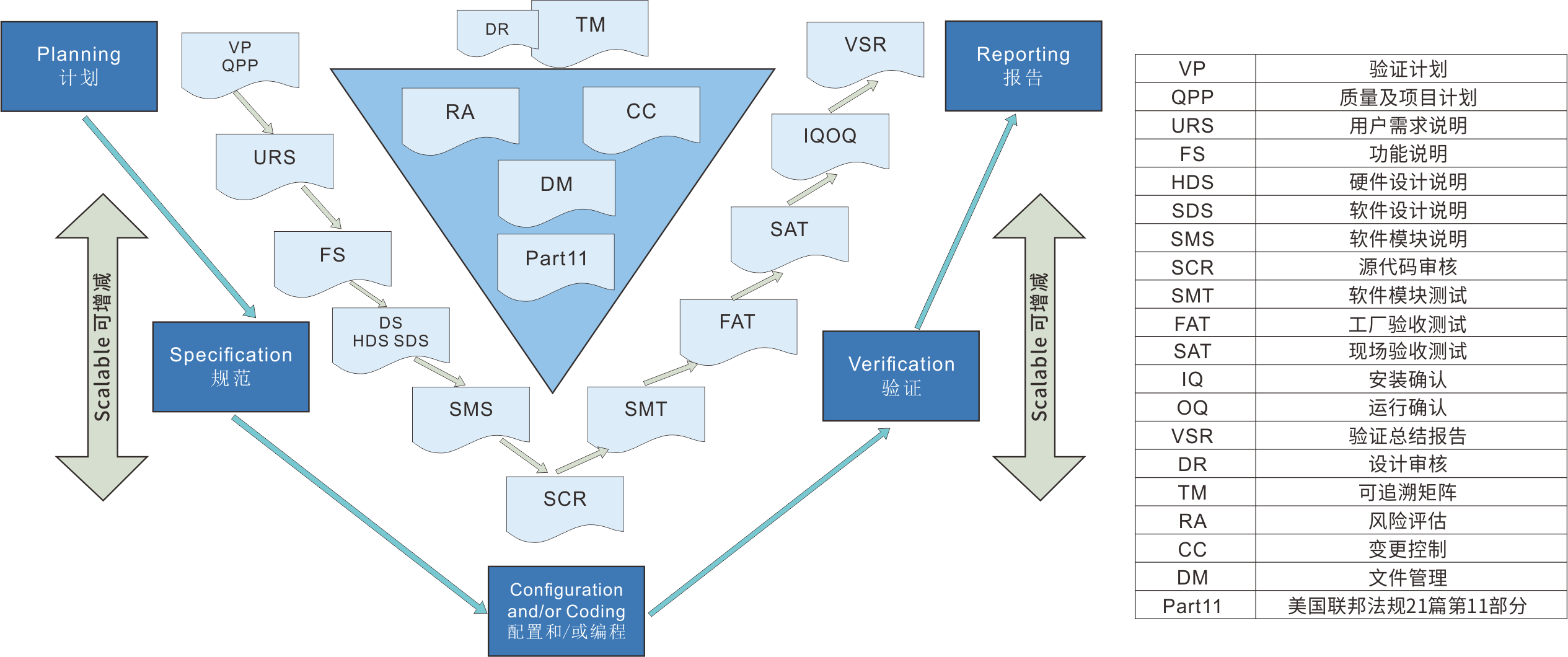
Computerized system validation
Computerized system verification is an important part of quality assurance in the pharmaceutical and related industries. We adhere to GXP calculations to ensure that the implementation and application of the system comply with regulatory consistency and risk management methods for data mechanization system supervision.
Reference regulations and guidelines
FDA 21 CFR Part 11 Electronic Records and Electronic Signatures.
Title 21, Part 210211 of the FDA Federal Regulations, Current Production Quality Management Practices for Finished Drugs.
EU Pharmaceutical Regulation Volume 4 GMP, Appendix 11 Computerized System.
2010 Revised GMP, Appendix 1 Computerized System, Appendix 2 Confirmation and Validation.
































 youtube
youtube



